43 open label extension study
Open-Label Extension Study | UPTRAVI® (selexipag) HCP In long-term follow-up of patients who were treated with UPTRAVI® in the placebo-controlled study (N=574) and the open-label extension study (N=330, of 574), Kaplan-Meier estimates of survival at 1, 2, 5, and 7 years were 92%, 85%, 71%, and 63%, respectively. Open label extension studies and patient selection biases Methods The usual method of analysis of the open label extension study, which ignores any patients not continuing into the follow-on period of the study, is outlined. It is shown that ignoring...
Understanding Clinical Trial Terminology: What is an Open Label ... Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population.

Open label extension study
Open-label Extension Study of ADP101 - ClinicalTrials.gov Detailed Description: This study is enrolling participants by invitation only. This is a multicenter, open-label, long-term extension study of the safety and efficacy of ADP101 for oral immunotherapy in food allergic children and adults who completed the Harmony study (protocol ADP101-MA-01). Study Design Go to Arms and Interventions Go to Consent to open label extension studies: some ethical issues A frequent feature of pharmaceutical research is the open label extension study, in which patients participating in double blind placebo controlled trials of new medications are invited, on completion of the initial trial, to take the study drug for some further period. An Open-label Extension Trial to Evaluate the Long-term Safety of ... None (Open Label) Primary Purpose: Treatment: Official Title: An Open-label Extension Trial to Evaluate the Long-term Safety of KVD900, an Oral Plasma Kallikrein Inhibitor, for On-demand Treatment of Angioedema Attacks in Adolescent and Adult Patients With Hereditary Angioedema Type I or II: Estimated Study Start Date : August 22, 2022
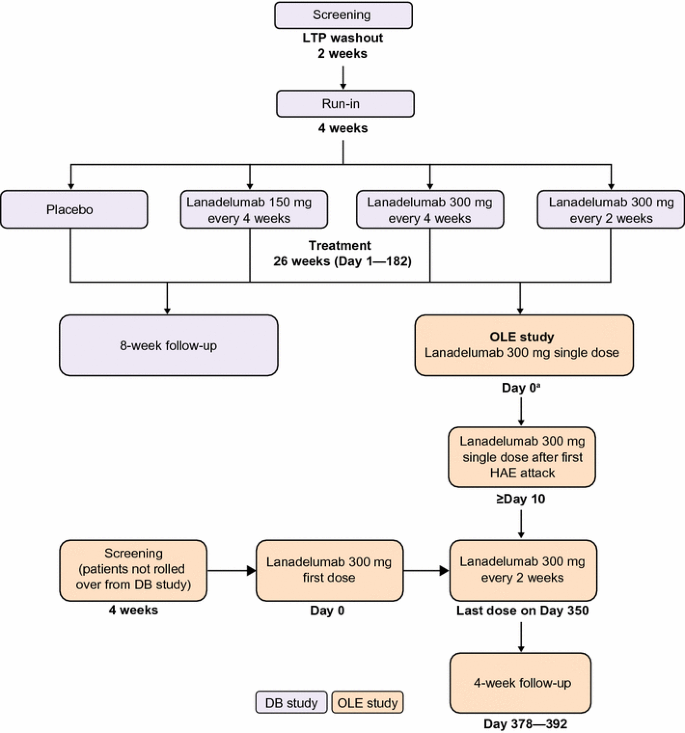
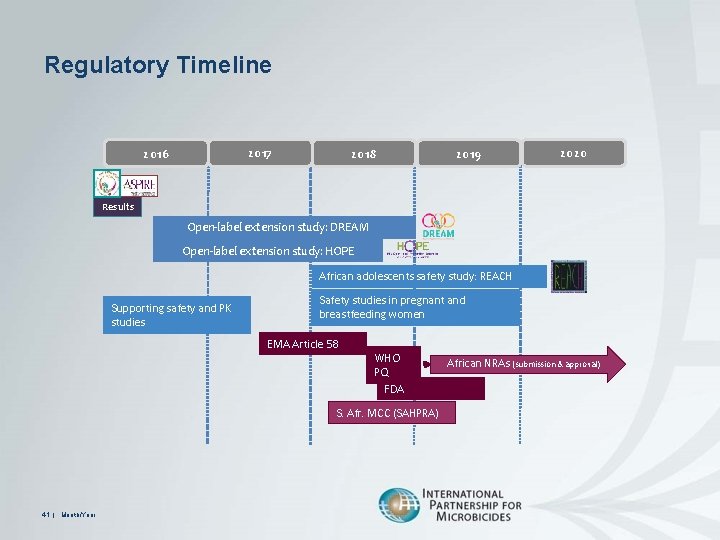
Open label extension study. Appendix 6 Summary of the Extension Study - NCBI Bookshelf Study CS3 is an ongoing open-label extension study. Its primary objective is to evaluate the safety of inotersen in patients with hereditary transthyretin-mediated amyloidosis (hATTR) with polyneuropathy. 51 The information summarized in this appendix is based on a planned interim analysis that included data up to February 28, 2017. Amgen to Present New Data at Esc Congress 2022 Highlighting Up to 8.5 ... In an open-label extension study in 106 patients, including 14 pediatric patients, no new adverse reactions were observed. Immunogenicity: Repatha is a human monoclonal antibody. As with all ... Safety and effectiveness of ulotaront (SEP-363856) in ... - Nature The aim of this 26-week open-label extension study was to evaluate the safety and effectiveness of ulotaront (25/50/75 mg/d) in patients who completed the initial 4-week study. Of the 193 4-week... Long-term safety and efficacy of dupilumab in patients with ... - PubMed This study aimed to evaluate the long-term safety and efficacy of dupilumab in patients with moderate-to-severe asthma, as data for extended treatment with dupilumab beyond 1 year are not available. Methods: The primary endpoint was the number and percentage of patients with any treatment-emergent adverse events.
Spotlight on Open-Label Extension Studies - Applied Clinical Trials Online Open-label extension (OLE) studies are common, but they do not receive as much attention as traditional Phase I through Phase IV studies. Enrollment into an OLE study typically follows enrollment into a randomized, blinded, well-controlled main study. Amgen Announces Results From Two Open Label Extension Studies of ... In an open-label extension study in 106 patients, including 14 pediatric patients, no new adverse reactions were observed. Immunogenicity: Repatha® is a human monoclonal antibody. Open-label extension studies: do they provide meaningful ... - PubMed Consumers, institutions where these studies are conducted and research ethics committees need to be convinced of the motives, as well as the quality, of the open-label extension study and its execution before supporting such studies. Open-label extension studies do have a legitimate but limited place in the clinical development of new medicines. What is an open label extension study? • NCK Pharma / Tags What is an open label extension study? An open - label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered.
An Open-Label Extension Study to Assess the Long-Term Efficacy and Saf ... The interventional, open-label extension study, SWIFT-VWDext (NCT01224808), was conducted in six centers in Bulgaria, Germany, Russia, Ukraine [one center each], and Poland [two centers] between October 2010 and March 2014. Avidity Biosciences Enrolls Patients in the MARINA™ Open-Label ... Patients have the option to enroll in MARINA-OLE, an open label extension study, at the end of the post-treatment period. In the fourth quarter of 2022, Avidity plans to conduct a preliminary assessment of safety, tolerability and key biomarkers in approximately half of the study participants in the MARINA study. End of Trial and Open-Label Extension (OLE) Frequently Asked Questions ... Open Label Extension, or OLE, is a phase of a study that occurs after the randomized (blinded) portion of the trial is completed if a drug is found to have the potential for benefit. Eligible trial participants take the active form of the drug without placebo. OLE allows active drug to be given to all participants at the same time and to follow them over time. Open-label Extension Study Evaluating Long-term Safety and Efficacy of ... Objective: This study sought to evaluate the long-term safety and efficacy of FMX101 4% topical minocycline foam for the treatment of moderate-to-severe acne.Design: This was an open-label extension of two double-blind studies, Study 04 and Study 05.Setting: Subjects were enrolled at 35 sites in the United States and one site in the Dominican Republic.
Use Extension Studies To Enhance Phase 3 Data - Clinical Leader "An open-label study is still technically a Phase 3 study, but the focus is on collecting more rigorous information on the long-term safety and tolerability of a new drug," says Parekh. "One of these studies would typically follow one or more randomized, double-blind clinical trials once the patient has completed treatments.
PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology of prolonged open-label extension. For example, the study of prednisolone remained randomized for 2 years2. The safety issues do not constitute a sufficient reason for con-ducting open-label extension studies. The third purpose may be to demonstrate continued effi-cacy of the drug over a longer period of time or to show that
Lipocine Announces Positive LPCN 1144 NASH Open Label Extension Study ... Lipocine Announces Positive LPCN 1144 NASH Open Label Extension Study Results LPCN 1144 was well tolerated over 72-week exposure with no observed safety signals Liver injury markers were reduced and maintained with extended LPCN 1144 treatment Observed liver histology improvements support further development
Open-Label Extension Studies | SpringerLink As the name implies, an open-label extension study is an 'appendage' to a randomised controlled clinical trial, usually of an unregistered medicine or intervention. Often the drug is being studied under an investigational new drug (IND) licence or equivalent legislation. The open-label extension study is identified formally as a study.
Open Label Extension Study Definition | Law Insider open label extension study means the open label extension portion ( to the extent relating to north america) of that certain phase iiia clinical study titled "cetrorelix pamoate intermittent im dosage regimens in patients with symptomatic bph: a 1 year placebo - controlled efficacy study and long -term safety assessment ," identified by protocol …
Open-label Extension Study of Efficacy, Safety and Tolerability of ... Open-label Extension Study of Efficacy, Safety and Tolerability of Secukinumab in Patients With Active Lupus Nephritis The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U.S. Federal Government.
Understanding Clinical Trial Terminology: What is a Long-Term Extension ... There is another type of study that exists between the traditional clinical trial phases and application for approval of a new medication: an open-label extension (OLE) study, sometimes also called a long-term extension study.
Open-Label Safety Extension study - Pfizer pro The open-label extension study did not evaluate the efficacy in EUCRISA. Open-Label Safety Extension (AD-303) 4. Participants in Trials 1 and 2 were given the option of participating in a long-term, open-label, single-arm safety study for 48 weeks; patients from both the EUCRISA and Emollient-rich Vehicle arms of the pivotal trials were ...
Pos0689 a 6-month Open-label Extension Study of The Safety and Efficacy ... Background: BLISS-LN (GSK Study BEL114054; [NCT01639339][1]), the largest lupus nephritis (LN) study to date, showed that intravenous (IV) belimumab (BEL) + standard therapy (ST) improved outcomes compared with ST alone in patients (pts) with active LN.1 Objectives: To assess additional safety and efficacy data of BEL + ST in pts with LN in a 6-month open-label (OL) phase beyond 2 years of ...
National Center for Biotechnology Information National Center for Biotechnology Information
An Open-label Extension Trial to Evaluate the Long-term Safety of ... None (Open Label) Primary Purpose: Treatment: Official Title: An Open-label Extension Trial to Evaluate the Long-term Safety of KVD900, an Oral Plasma Kallikrein Inhibitor, for On-demand Treatment of Angioedema Attacks in Adolescent and Adult Patients With Hereditary Angioedema Type I or II: Estimated Study Start Date : August 22, 2022
Consent to open label extension studies: some ethical issues A frequent feature of pharmaceutical research is the open label extension study, in which patients participating in double blind placebo controlled trials of new medications are invited, on completion of the initial trial, to take the study drug for some further period.
Open-label Extension Study of ADP101 - ClinicalTrials.gov Detailed Description: This study is enrolling participants by invitation only. This is a multicenter, open-label, long-term extension study of the safety and efficacy of ADP101 for oral immunotherapy in food allergic children and adults who completed the Harmony study (protocol ADP101-MA-01). Study Design Go to Arms and Interventions Go to































Post a Comment for "43 open label extension study"