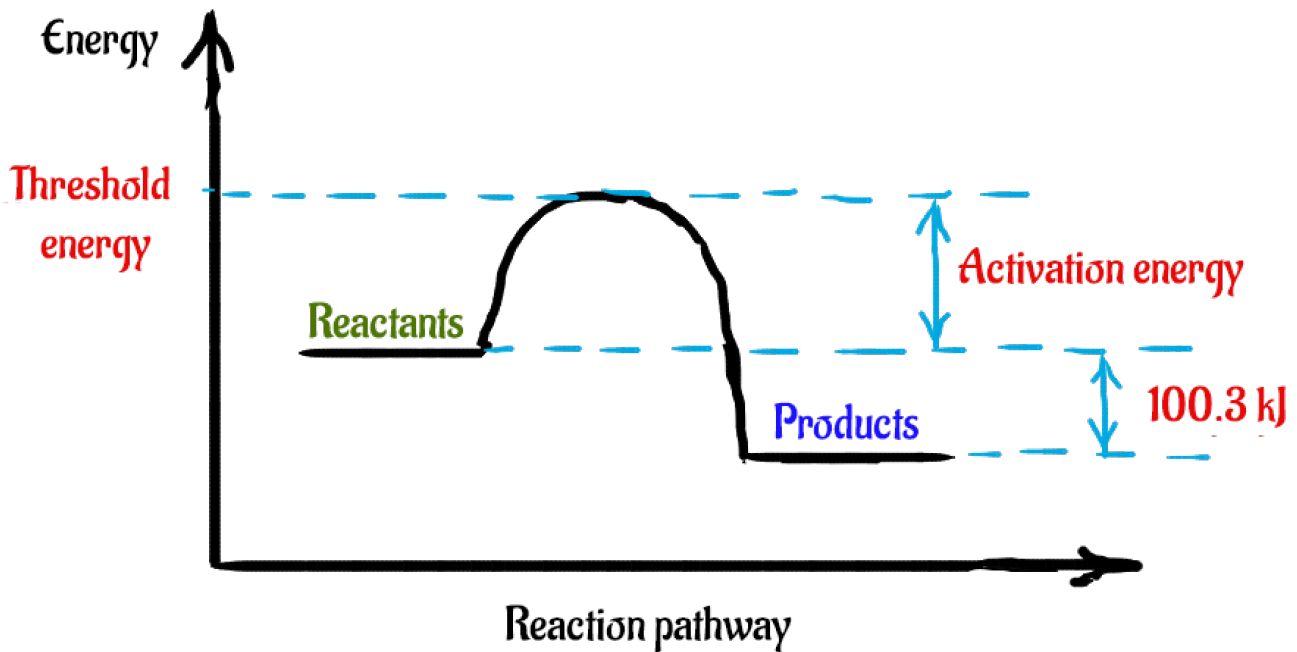
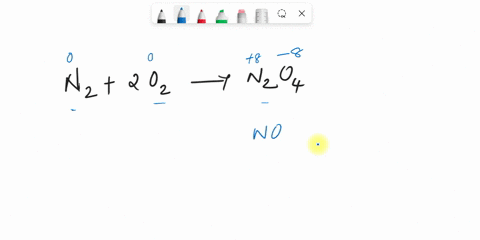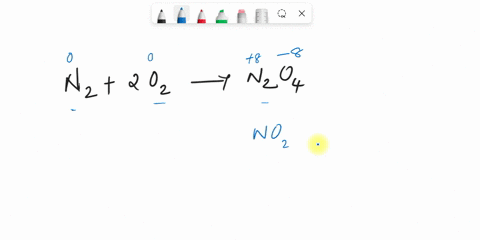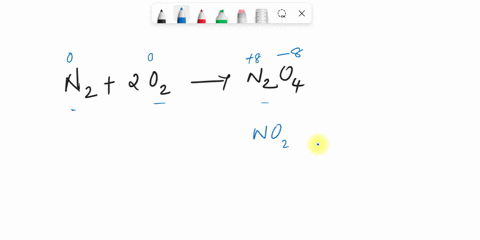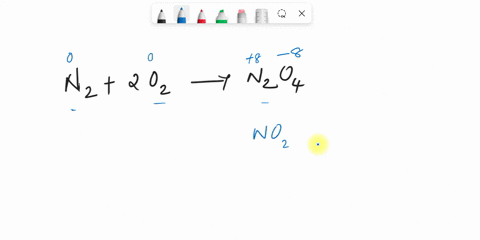
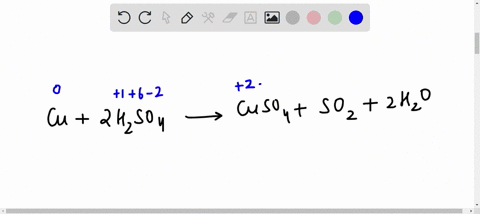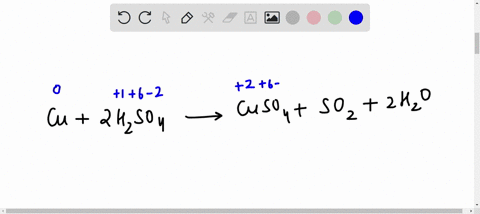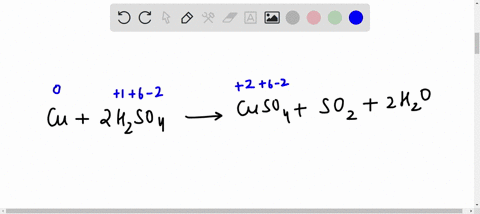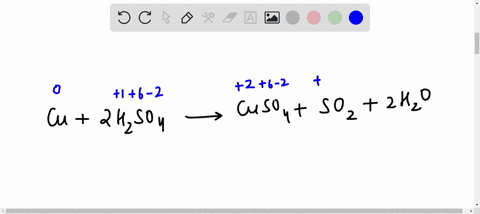
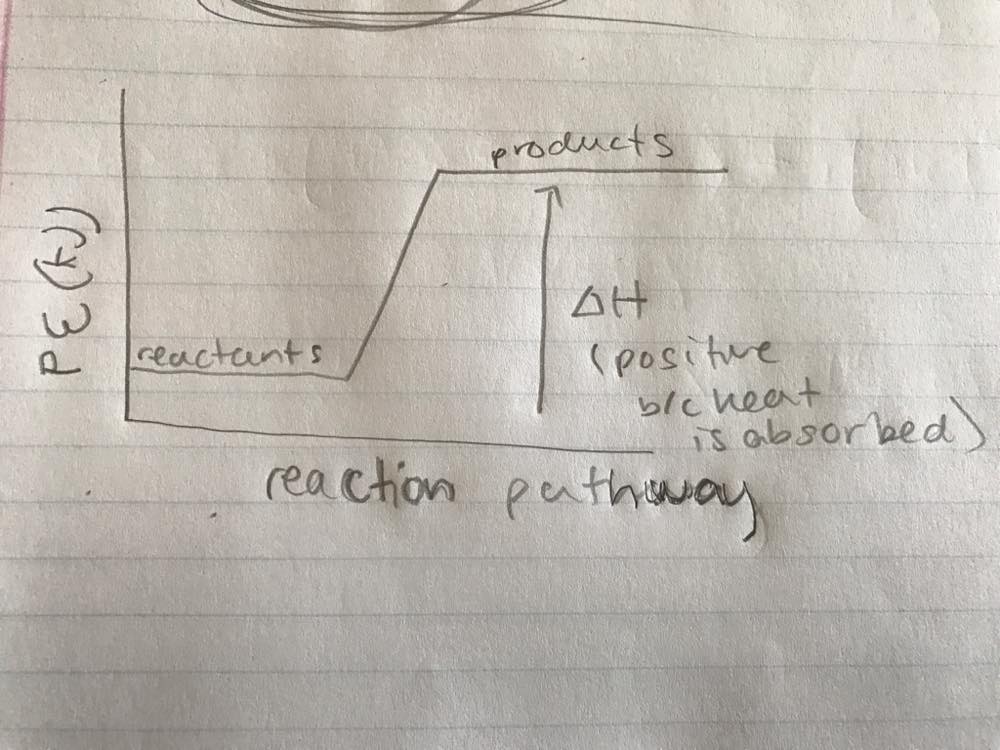
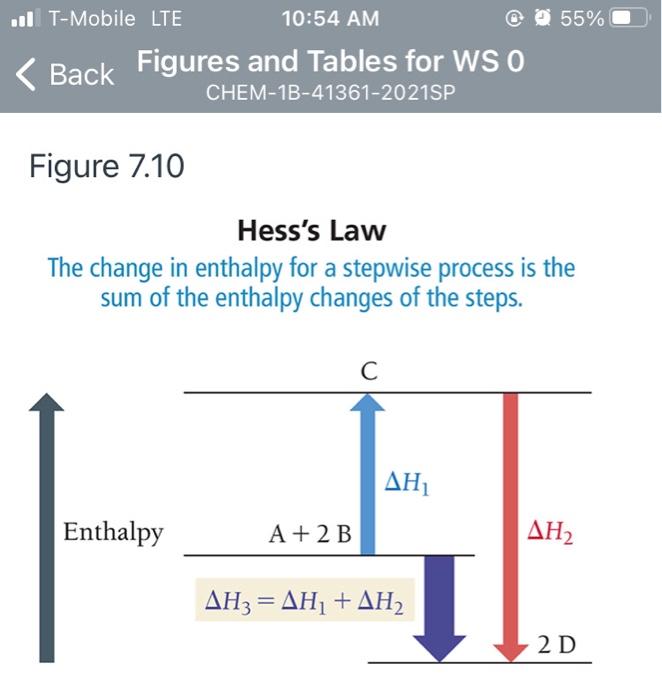
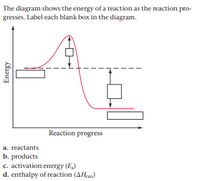
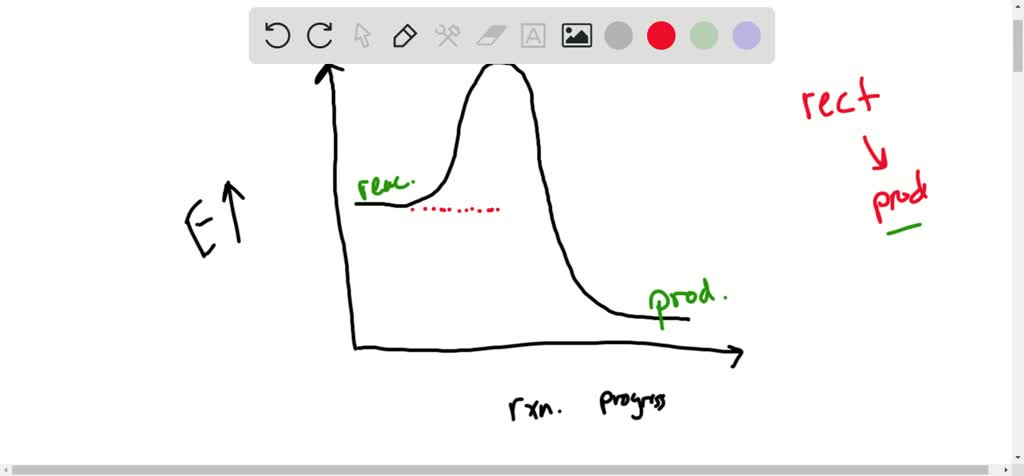
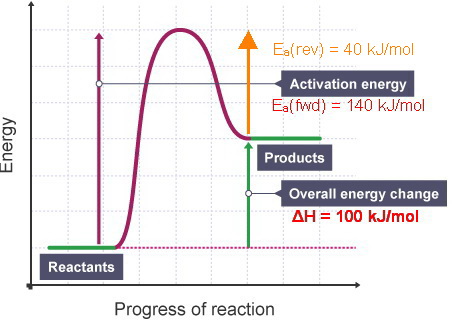
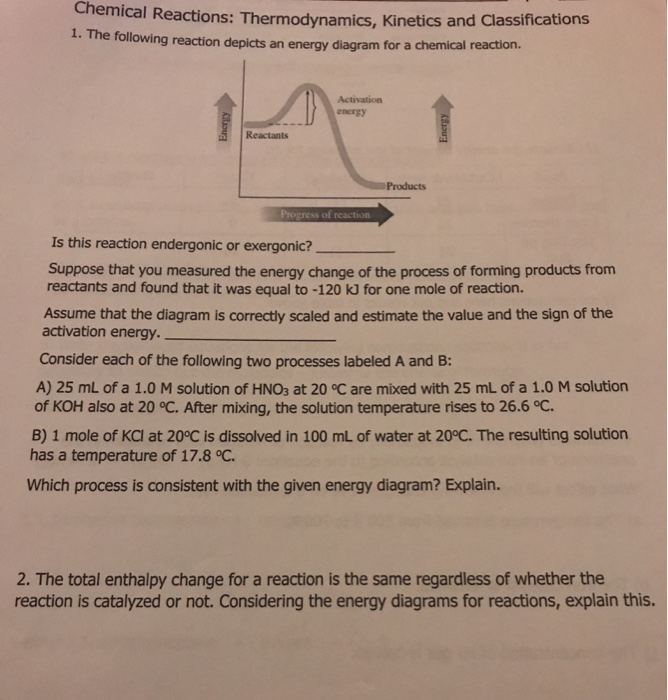
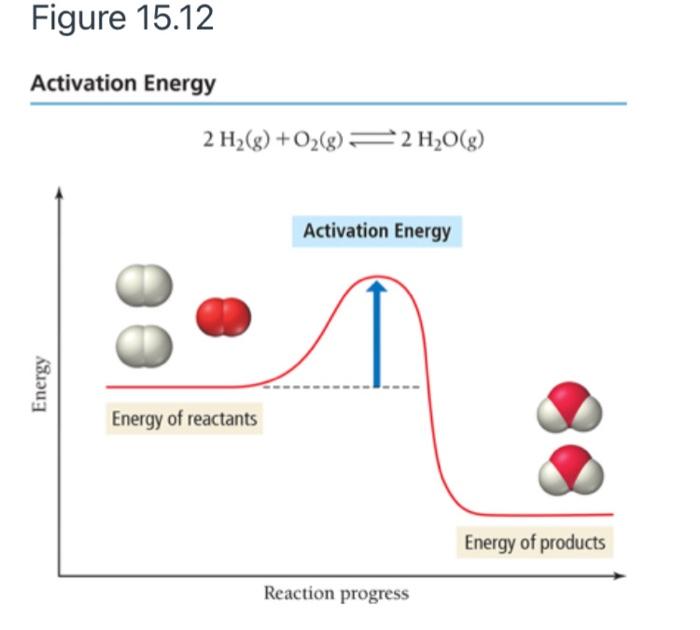
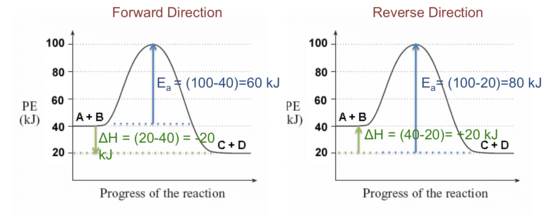
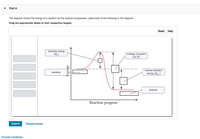
44 label the reactants and products on the enthalpy diagrams for each process.
IB Diploma Chemistry HL Textbook pdf - Academia.edu Titration is the process in which the standard reagent is added to a solution of the sample until the reaction is judged to be complete. Back titration is the process by which the excess of the standard solution used to consume the sample is determined by titration with a second standard solution. CHAPTER 1 CHEMISTRY: THE STUDY OF CHANGE Problem Categories Enter the email address you signed up with and we'll email you a reset link.
Chemical formula - Wikipedia The term empirical refers to the process of elemental analysis, a technique of analytical chemistry used to determine the relative percent composition of a pure chemical substance by element. For example, hexane has a molecular formula of C 6 H 14 , and (for one of its isomers, n-hexane) a structural formula CH 3 CH 2 CH 2 CH 2 CH 2 CH 3 ...
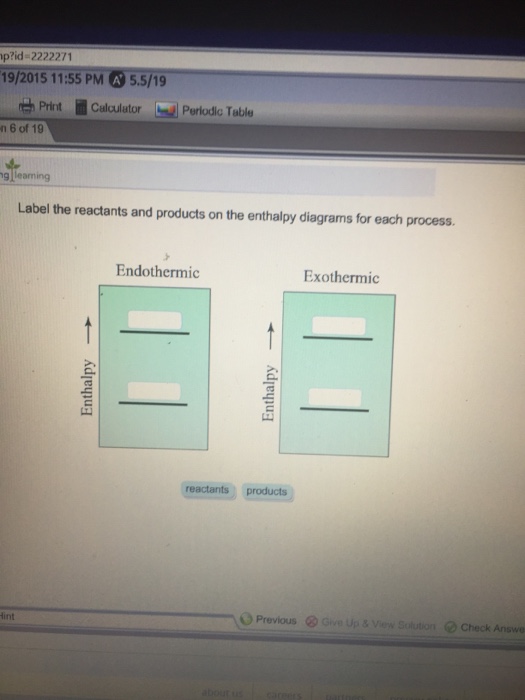
Label the reactants and products on the enthalpy diagrams for each process.
5.2 Calorimetry - Chemistry 2e | OpenStax Figure 5.17 (a) A bomb calorimeter is used to measure heat produced by reactions involving gaseous reactants or products, such as combustion. (b) The reactants are contained in the gas-tight “bomb,” which is submerged in water and surrounded by insulating materials. (credit a: modification of work by “Harbor1”/Wikimedia commons) Engineering Chemistry by Jain & Jain - Academia.edu Each system contains the thioester portion of acyl-CoA (CH3CH2(CO)SCH3) a weakly basic COO representing Glu376, H-donor molecules (methanol and methylformamide) with zero, one or two H-donors bound to the carbonyl oxygen of the thioester correspond to the Glu376 and FAD side-chains. CONCISE REVISION COURSE ® •CSEC ChEMISTRY BY Anne Tindale Titration is the process in which the standard reagent is added to a solution of the sample until the reaction is judged to be complete. Back titration is the process by which the excess of the standard solution used to consume the sample is determined by titration with a second standard solution.
Label the reactants and products on the enthalpy diagrams for each process.. FE Reference Handbook 10.0.1 - Academia.edu Enter the email address you signed up with and we'll email you a reset link. CONCISE REVISION COURSE ® •CSEC ChEMISTRY BY Anne Tindale Titration is the process in which the standard reagent is added to a solution of the sample until the reaction is judged to be complete. Back titration is the process by which the excess of the standard solution used to consume the sample is determined by titration with a second standard solution. Engineering Chemistry by Jain & Jain - Academia.edu Each system contains the thioester portion of acyl-CoA (CH3CH2(CO)SCH3) a weakly basic COO representing Glu376, H-donor molecules (methanol and methylformamide) with zero, one or two H-donors bound to the carbonyl oxygen of the thioester correspond to the Glu376 and FAD side-chains. 5.2 Calorimetry - Chemistry 2e | OpenStax Figure 5.17 (a) A bomb calorimeter is used to measure heat produced by reactions involving gaseous reactants or products, such as combustion. (b) The reactants are contained in the gas-tight “bomb,” which is submerged in water and surrounded by insulating materials. (credit a: modification of work by “Harbor1”/Wikimedia commons)
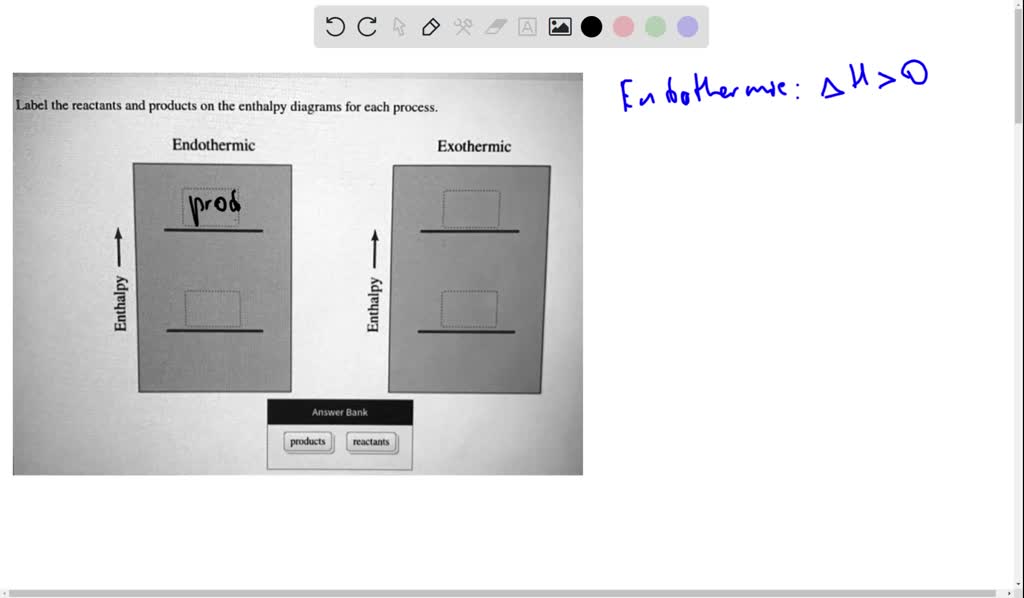
Label the reactants and products 0 the enthalpy diagrams for each process, Endothermic, Exothermic, J, Answer Bank, products, maclnm, j




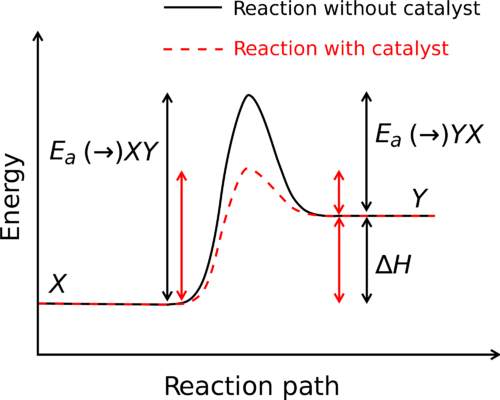
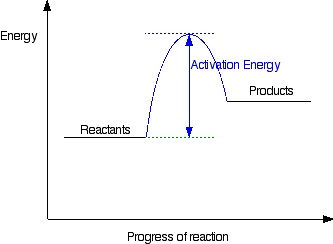



















Post a Comment for "44 label the reactants and products on the enthalpy diagrams for each process."