44 how to cite fda package insert apa
How do I cite a drug label? - Ask HSL In AMA: Lamasil [package insert]. East Hanover, NJ: Sandoz Pharmaceuticals Corp; 1993. In APA: Sandoz Pharmaceuticals Corp. (1993). Lamasil [package insert]. Package Insert - Pharmacy - Citing Resources Guide - FDU LibGuides Dec 9, 2022 ... City, State Abbreviation: Manufacturer's Name; Year. Example: Byetta(R) [package insert]. San Diego, CA: Amylin Pharmaceuticals Inc; 2007.
Package Inserts - Vancouver Citation Style Guide Aug 14, 2022 ... Drug name [package insert]. Place of publication: Manufacturer; publication year. Example of a Package Insert Citation.
How to cite fda package insert apa
Package Insert - Reference Guide for Pharmacy Students Mar 9, 2023 ... Example: Byetta. Package insert. Amylin Pharmaceuticals Inc; 2007. APA Style - Citation Format Jan 3, 2023 ... Author. (Year). Drug: Article TItle. Publisher. Shionogi Pharma. (2010). Ulesfia Lotion: Highlights of prescribing information ... How to Cite a Package Insert: 9 Steps (with Pictures) - wikiHow Type the name of the manufacturer followed by a comma and the year the package insert was published. [6] X Research source. Example: (Merck Sharp & Dohme Corp., ...
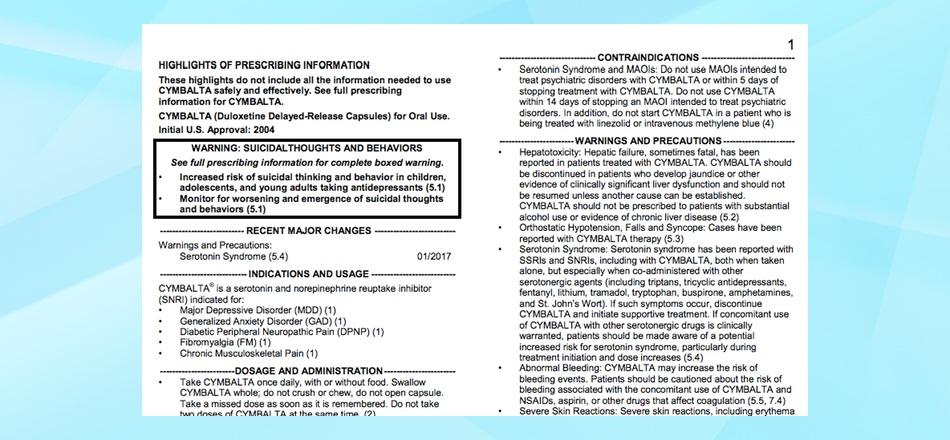
How to cite fda package insert apa. Package Inserts - Citation Guide: ICMJE - LibGuides at PCOM Library Nov 15, 2022 ... Drug name [package insert]. Place of publication: Manufacturer's name; Year of publication. Example Albuterol [package insert]. West Roxbury, MA ... Q. How do I cite a drug package insert? May 22, 2019 ... Manufacturer. Name of medicine [package insert]. U.S. Food and Drug Administration website. URL. Revised [date]. Accessed [date]. For example ... Citation Format: AMA Style - LibGuides Jan 3, 2023 ... Lamasil [package insert]. East Hanover, NJ: Sandoz Pharmaceutics Corporation; 1993. Package Inserts - online. Drug name ... A Prescription for Success: How to Cite Product Information in APA ... May 31, 2012 ... What is the document called? The title at the top of the insert (Highlights of Prescribing Information) is not too informative, but together ...
How to Cite a Package Insert: 9 Steps (with Pictures) - wikiHow Type the name of the manufacturer followed by a comma and the year the package insert was published. [6] X Research source. Example: (Merck Sharp & Dohme Corp., ... APA Style - Citation Format Jan 3, 2023 ... Author. (Year). Drug: Article TItle. Publisher. Shionogi Pharma. (2010). Ulesfia Lotion: Highlights of prescribing information ... Package Insert - Reference Guide for Pharmacy Students Mar 9, 2023 ... Example: Byetta. Package insert. Amylin Pharmaceuticals Inc; 2007.
















![Institution Logo]](https://s2.studylib.net/store/data/017675115_1-9b9d82836b75eed10a9c73f3d8ec0b7a.png)


















Post a Comment for "44 how to cite fda package insert apa"