45 how to cite fda drug label
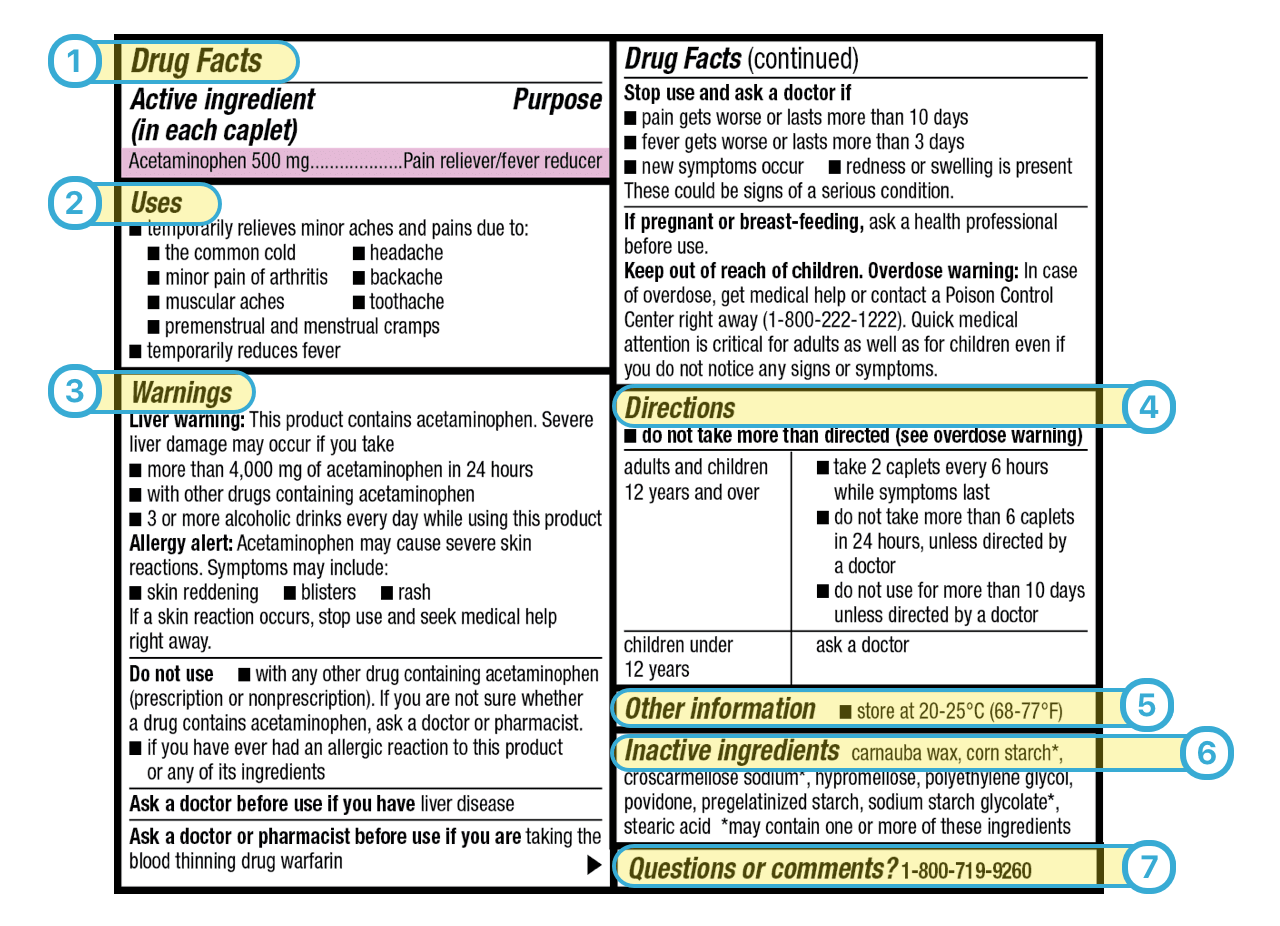
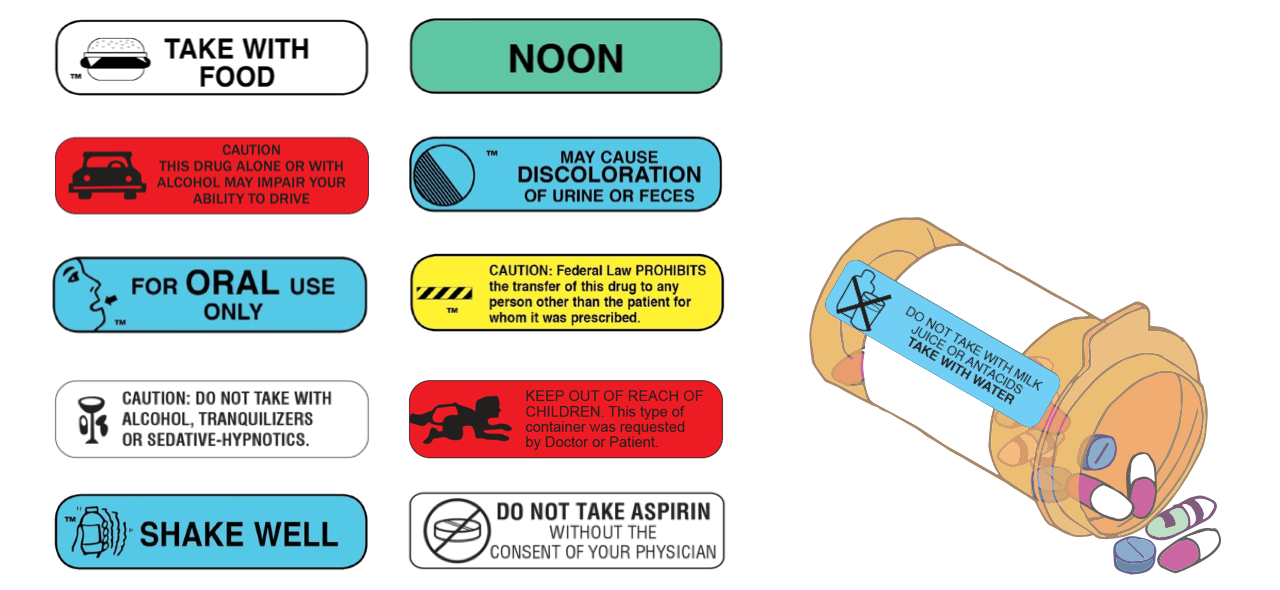
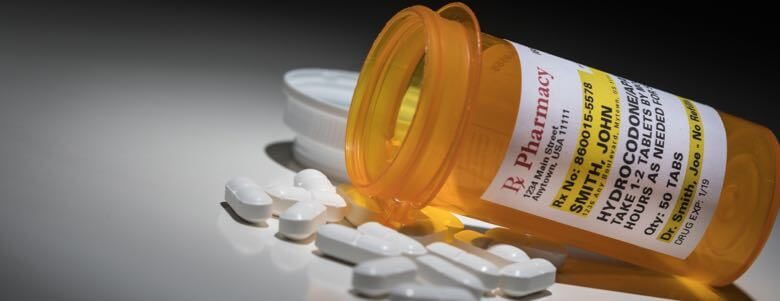
How to Read Over-the-Counter and Prescription Drug Labels Check the label to make sure your name is on it. If it isn't, talk to the pharmacist. Check the label to make sure you can read and understand the name of the medicine, directions and colored warning stickers on the package. If the letters are too small to read, ask your pharmacist to print it in a larger type. PDF Citing FDA Approval Letters - Jane Ganter Citing FDA Approval Letters Citing electronic sources can tax the capacity of an obedient author to conform to the style manual. But I rely on, and take comfort from, passages in APA's Publication Manual like this one (p. 269). The variety of material available on the Web, and the variety of ways in which it is structured
FDA Label Search-Application Number FDA Label Search. FDA Home - Search by Application Number or Regulatory Citation: ... including the leading zero. For citations, type in "part" and at least a portion of the citation (e.g., part310)" Return to the FDA Label Search Page - - Links on this page: ... U.S. Food and Drug Administration. 10903 New Hampshire Avenue Silver Spring, MD ...
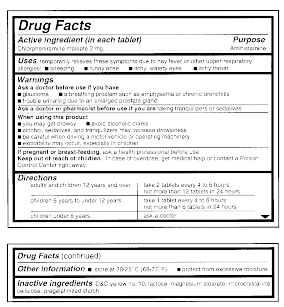
How to cite fda drug label
Drug Label Annotations - PharmGKB PharmGKB annotates drug labels containing pharmacogenetic information approved by the US Food and Drug Administration (FDA), European Medicines Agency (EMA), Swiss Agency of Therapeutic Products (Swissmedic), Pharmaceuticals and Medical Devices Agency, Japan (PMDA) and Health Canada (Santé Canada) (HCSC).. PharmGKB annotations provide a brief summary of the PGx in the label, an excerpt from ... Labeling Information | Drug Products | FDA For prescription drug labeling resources (e.g., Prescribing Information, FDA-approved patient labeling, and carton and container labeling), please see the Prescription Drug Labeling Resources web page Introduction to the New Prescription Drug Labeling by the FDA A prescription drug product label (also known as a professional label, package insert, direction circular, and package circular) is a compilation of information about a product written by the...
How to cite fda drug label. Outdated Prescription Drug Labeling: How FDA-Approved Prescribing ... We compared the number of drug uses indicated on product labels to the number of uses contained in a leading drug compendium for 43 cancer drugs approved between 1999 and 2011. We defined a "well-accepted off-label use" of a drug as one that was not approved by the FDA and received a category 1 or 2A evidence grade. Labeling for Human Prescription Drug and Biological Products ... FDA is issuing this guidance to provide recommendations for applicants developing labeling for new prescription drugs and revising labeling for already approved prescription drugs. This guidance... FDA's Labeling Resources for Human Prescription Drugs Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective use of the drug; and (2) includes the Prescribing Information,... FDA Label Search The drug labels and other drug-specific information on this Web site represent the most recent drug listing information companies have submitted to the Food and Drug Administration (FDA). (See 21 CFR part 207.) ... Application Number or Regulatory Citation Search: Company Search: Proprietary Name and Company Search:
Fine-Tuning BERT for Automatic ADME Semantic Labeling in FDA Drug ... Product-specific guidances (PSGs) recommended by the United States Food and Drug Administration (FDA) are instrumental to promote and guide generic drug product development. To assess a PSG, the FDA assessor needs to take extensive time and effort to manually retrieve supportive drug information of absorption, distribution, metabolism, and excretion (ADME) from the reference listed drug ... Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 314.3 Definitions. (a) The definitions and interpretations contained in section 201 of the Federal Food, Drug, and Cosmetic Act apply to those terms when used in this part and part 320 of this chapter. How Do I Use Prescription Drug Labeling | FDA FDA-Approved Patient Labeling Patient labeling may be physically attached or provided separately from the USPI and contains information in lay language that can help patients use a drug safely and... FDALabel: Full-Text Search of Drug Product Labeling | FDA Because the SPL documents on FDALabel may not be identical to the most recent FDA-approved labeling, visit the following sites for the most current FDA-approved labeling: Drugs@FDA for human...
FDALabel - Bioinformatics Tools | FDA Labeling, Product and Ingredient Identifiers. Application Number for ANDA, BLA, or NDA: 3 to 6 digits (e.g., 077844, 125118, 020977) Unique Ingredient Identifier (UNII): To search for active ingredients, inactive ingredients or both, type in alphanumeric code (s) (e.g., J220T4J9Q2) Referencing/Citing Drugs.com Subscribe to Drugs.com newsletters for the latest medication news, new drug approvals, alerts and updates. Drugs.com provides accurate and independent information on more than 24,000 prescription drugs, over-the-counter medicines and natural products. FDA Guidance on Differences Between RLD and Reference Standard for ANDA ... Ordinarily, the reference standard selected by FDA will be the RLD; however, that is not always so. If FDA has selected a reference standard for use in in vivo bioequivalence studies different from the RLD, then the ANDA applicant must compare its proposed product's labeling and formulation to that of the RLD and not to the reference standard. How to Cite a Package Insert: 9 Steps (with Pictures) - wikiHow Provide the name of the drug and title of the package insert in italics. Type the full name of the drug followed by a colon. Then type the title of the package insert as stated at the top of the insert. Type the title in sentence-case, capitalizing only the first word and any proper nouns. Place a period at the end of the title. [4]
Federal Register :: Revising Abbreviated New Drug Application Labeling ... Submit written requests for single copies of the draft guidance to the Division of Drug Information, Center for Drug Evaluation and Research, Food and Drug Administration, 10001 New Hampshire Ave., Hillandale Building, 4th Floor, Silver Spring, MD 20993-0002. Send one self-addressed adhesive label to assist that office in processing your requests.
What are the FDA Labeling Requirements for Cosmetic Products? This Act requires that all consumer commodities should be labeled in a way that reveals the amount of product in the package, the identity of the commodity, as well as the name/place of business of the product's manufacturer. The Act also allows for additional regulations to prevent other forms of consumer deception due to labeling.
Drugs@FDA: FDA-Approved Drugs For prescription brand-name drugs, Drugs@FDA typically includes the most recent labeling approved by the FDA (for example, Prescribing Information and FDA-approved patient labeling when available),...
Reference Listed Drug, RLD, ANDA, Generic drug, FDA A Reference Listed Drug (RLD), as goes by its innate meaning, is an FDA approved drug product which can be referred to by a generic drug manufacturer while filing an Abbreviated New Drug Application (ANDA). An RLD is basically useful to establish bioequivalence of the product with that of an already approved one.
Code of Federal Regulations Title 21 - Food and Drug Administration The information on this page is current as of Jan 06, 2022. For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 201.20 Declaration of presence of FD&C Yellow No. 5 and/or FD&C Yellow No. 6 in certain drugs for human use. (a) The label for over-the-counter and prescription drug products ...
FDA Issues New Guidance on Updating Labeling for Generics After ... January 26, 2022 Pharmaceuticals Regulatory Affairs The FDA has released draft guidance for applicants and holders of an abbreviated new drug application (ANDA) on updating their labeling after revisions to the approved labeling of a reference listed drug (RLD) on which a generic drug is based. To View This Article: Login
Reference Listed Drug, Reference Standard, Basis of Submission - FDA ... As we know, all drug products approved for safety or efficacy are cited in the Orange Book and are considered "listed" drugs. The FDA now is making a distinction between the designation of an RLD (the drug upon which an ANDA may be based) and a reference standard (the drug that FDA expects the firm to use for establishing bioequivalence).
Code of Federal Regulations Title 21 - Food and Drug Administration The requirements in this section apply only to prescription drug products described in § 201.56(b)(1) and must be implemented according to the schedule specified in § 201.56(c), except for the requirement in paragraph (c)(18) of this section to reprint any FDA-approved patient labeling at the end of prescription drug labeling or accompany the ...
FDA Drug Label Data - CKAN Metadata Updated: April 25, 2021. This file contains the data elements used for searching the FDA Online Data Repository including proprietary name, active ingredients, marketing application number or regulatory citation, National Drug Code, and company name.
Code of Federal Regulations Title 21 - Food and Drug Administration (a) The immediate package of an investigational new drug intended for human use shall bear a label with the statement "Caution: New Drug - Limited by Federal (or United States) law to...
FDA Drug Registration | FDA Drug Listing | Drug Labeling Requirements ... A Private Label Distributor (PLD) does not require US FDA drug registration. A contract sterilizer and Contract Testing Laboratory (dosage forms & active ingredient release) require US FDA registration but not listing. A foreign drug manufacturer also requires US FDA registration and listing, if drug from the manufacturer is marketing in the USA.
Introduction to the New Prescription Drug Labeling by the FDA A prescription drug product label (also known as a professional label, package insert, direction circular, and package circular) is a compilation of information about a product written by the...
Labeling Information | Drug Products | FDA For prescription drug labeling resources (e.g., Prescribing Information, FDA-approved patient labeling, and carton and container labeling), please see the Prescription Drug Labeling Resources web page
Drug Label Annotations - PharmGKB PharmGKB annotates drug labels containing pharmacogenetic information approved by the US Food and Drug Administration (FDA), European Medicines Agency (EMA), Swiss Agency of Therapeutic Products (Swissmedic), Pharmaceuticals and Medical Devices Agency, Japan (PMDA) and Health Canada (Santé Canada) (HCSC).. PharmGKB annotations provide a brief summary of the PGx in the label, an excerpt from ...

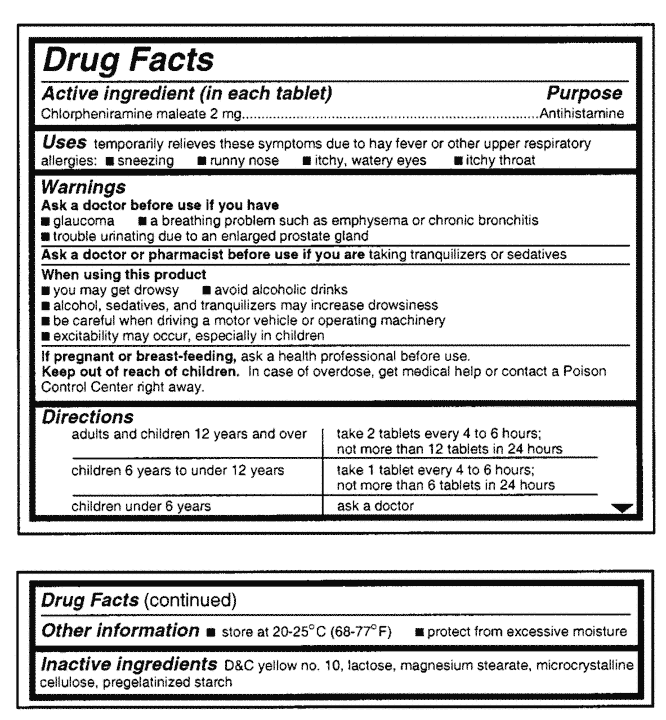
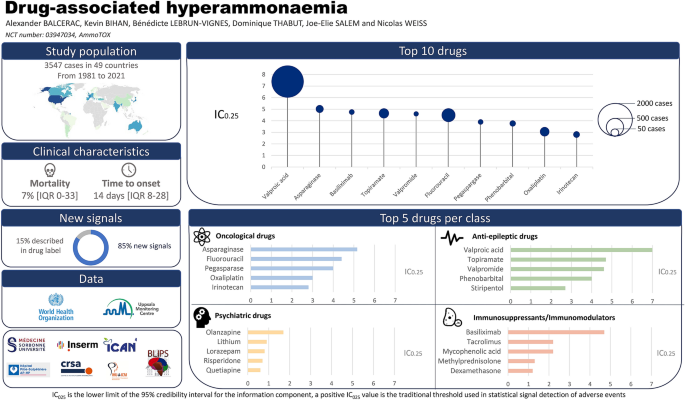






















![PDF] Toward Creating a Gold Standard of Drug Indications from ...](https://d3i71xaburhd42.cloudfront.net/c231e1899261c9d123b8e45ed53703ebbe47f74b/2-Figure1-1.png)

Post a Comment for "45 how to cite fda drug label"