38 what term is used to label the energy levels of electrons
Energy levels | Article about Energy levels by The Free Dictionary The lowest level ℰ 0, which corresponds to the lowest possible energy of the system, is called the ground state; all other energy levels ℰ 1, ℰ 2, . . . are called excited states, since the system must Figure 1 be excited, that is, must acquire energy, to undergo a transition to these levels. Energy level - Wikipedia The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can also refer to energy levels of nuclei or vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be quantized .
why the energy levels are named as k l m n and not as a b c d in an ... Therefore, it was decided that the K type X-ray is the highest energy X-ray an atom can emit. It is produced when an electron in the innermost shell is knocked free and then recaptured. This innermost shell is now called the K-shell, after the label used for the X-ray. He also won the 1917 Nobel Prize for Physics for this work.

What term is used to label the energy levels of electrons
What term is used to label the energy levels of electrons? The term that is used to label the energy levels of electrons are principle quantum numbers and a valance band refers to the "energy levels in an atom where the electrons that participate in bonding occupy. These energy levels correspond to those of the s and p orbitals of the outermost shell of the atom being considered." Hope this helps! :) s,p,d,f Orbitals - Chemistry | Socratic An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. The order of size is 1s < 2s < 3s < …, as shown below. Chapter 5 test Section 5.1 Flashcards - Quizlet The term that is used to label the energy levels of electrons is? S. What letter is used to denote a spherical orbital? Dumbell. All "p" orbital's are _____ shaped? ... Sets with similar terms. Chemistry 101 Exam 1 (topics 1-8) Neyhart. 77 terms. mag998. unit 2: role of the electron. 49 terms. aneshaj01. Chem test 7. 41 terms. lexi_lyles6.
What term is used to label the energy levels of electrons. Electrons and Sublevels - Kentchemistry.com Theoretically there are an infinite number principal energy levels and sublevels. If you are just starting to study chemistry, you should only be concerned with the first 4 sublevels. Each sublevel is assigned a letter. The four you need to know are s (sharp), p (principle), d (diffuse), and f (fine or fundamental). So, s,p,d & f. chemistry ch. 5 section 1 Flashcards - Quizlet Review terms and definitions ... t/f, the electrons in an atom can exist between energy levels? ... used to label the energy levels of electrons Chapter 5 Electrons in Atoms Flashcards | Quizlet What is the term that is used to label the energy levels of electrons? principal quantum numbers (n). Chapter 5 Chem Flashcards | Quizlet Quantum of energy is the amount of energy required to Farther the higher the electron the blank it is from the nucleus Atomic Orbital often thought of as a region of space in which there is a high probability of finding an electron Principle quantum numbers the term used to label the energy levels of electrons S used to denotate a spherical orbital
What is the term used to label the energy levels of electron What is the term used to label the energy levels of electron 1 See answer Advertisement Greatanonymous09 Answer: The quantum mechanical model of the atom estimates the probability of finding an electron in a certain position. ... Circle the letter of the formula for the maximum number of electrons that can accupy a principal energy level. Define the following: a. main energy levels b. quantum numbers Main energy levels, or shell, represents a fixed distances from the nucleus where we can find electrons. It is symbolized by 'n', and as the value of 'n' ... PDF Electrons and The Structure of Atoms - Jefferson Academy Chemistry 9. Circle the letter of the term that is used to label the energy levels of electrons. atomic orbitals quantum mechanical numbers quantum principal quantum numbers (n) 10. The letter 11. Label each diagram below px, py, or is used to denote a spherical orbital. pz. p orbitals Energy Level and Transition of Electrons - Brilliant The energy of the electron of a monoelectronic atom depends only on which shell the electron orbits in. The energy level of the electron of a hydrogen atom is given by the following formula, where n n denotes the principal quantum number: E_n=-\frac {1312} {n^2}\text { kJ/mol}. E n = − n21312 kJ/mol.
How to Represent Electrons in an Energy Level Diagram Chemists use the energy level diagram as well as electron configuration notation to represent which energy level, subshell, and orbital are occupied by electrons in any particular atom. Chemists use this information in these ways: To predict what type of bonding will occur with a particular element and show exactly which electrons are being used Chapter 5 Chemistry Flashcards | Quizlet After discovering the atomic nucleus ______ used existing ideas about the atom and proposed ... term that is used to label the energy levels of electrons:. Energy level | Article about energy level by The Free Dictionary energy level. [ ′en·ər·jē ‚lev·əl] (geology) The kinetic energy supplied by waves or current action in an aqueous sedimentary environment either at the interface of deposition or several meters above. (quantum mechanics) An allowed energy of a physical system; there may be several allowed states at one level. What term is used to label the energy levels of electrons? - Answers What term is used to label energy levels of electrons? Principal quantum numbers (n). What is the term valance band? It refers to the energy levels in an atom where the electrons that participate...
What the Numbers on the Periodic Table Mean - ThoughtCo The period indicates the highest energy level attained by electrons of an atom of the element in the ground state. How to Identify It: Period numbers are located on the left-hand side of the table. These are simple integer numbers. Examples: The row starting with hydrogen is 1. The row starting with lithium is 2.
The periodic table, electron shells, and orbitals - Khan Academy Most of the elements important in biology need eight electrons in their outermost shell in order to be stable, and this rule of thumb is known as the octet rule. Some atoms can be stable with an octet even though their valence shell is the 3n shell, which can hold up to 18 electrons.
Energy Bands - Definition and Classification of Energy Bands - BYJUS The electrons in the same orbit exhibit different energy levels. The grouping of these different energy levels is known as the energy band. However, the energy of the inner orbit electrons is not much affected by the presence of neighbouring atoms. Classification of Energy Bands Valence Band
What is the term used to label the energy levels of electrons ... - Answers What term is used to label energy levels of electrons? Principal quantum numbers (n). What is the term valance band? It refers to the energy levels in an atom where the electrons that participate...
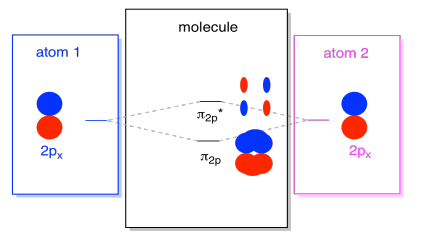
Energy level diagram for Molecular orbitals - Class Notes As no unpaired electron is present, the H2 molecule should be diamagnetic. 3) H2- The electronic configuration of H2- is ( σ (1s) )2 (σ∗(1s)) 1 1) Bond order= ½ 2) Smaller positive value of bond order indicates that it is somewhat stable. 3) Since it has one unpaired ,it is paramagnetic. 4) He2
Protons, Neutrons, and Electrons - Middle School Chemistry Students will be able to explain, in terms of electrons and protons, why a charged object is attracted or repelled by another charged object. ... It shows the electron in the space surrounding the nucleus that is called an electron cloud or energy level. It is not possible to know the location of an electron but only the region where it is most ...
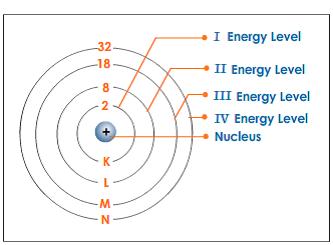
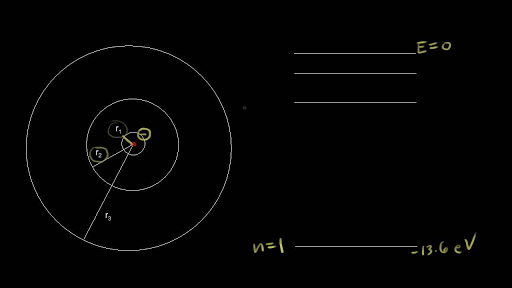
Energy Level of an Atom - Energy State and Energy level Diagrams - VEDANTU The energy levels are also called electron shells. An electron can move in one energy level or to another energy level, but it can not stay in between two energy levels. (Image will be uploaded soon) The figure shows the energy levels of an atom. The first four energy levels are shown here. The first energy level is also called level 'K'.
Energy Level Diagram - Different Energy Shells Around the Nucleus - BYJUS In chemistry, an electron shell, or energy level, may be imagined as an orbit with electrons around the nucleus of an atom. Bohr developed this model of the atom which says the electrons revolve around the nucleus in a circular path called an orbit.
Solved Molecule of study: K3[Co(C2O4)3] a) Write down the - Chegg c) Use orbital and symmetry symbols to label energy levels and assign electrons to the energy levels in your diagram, considering that this compound is paramagnetic. [5 marks] d) Clearly identify the Δo energy splitting transition on your MO diagram and explain your choice. Calculate the splitting energy in cm-1 knowing that λmax = 600 nm. [5 ...
What Is The Term Used To Label The Energy Levels Of ... Jul 21, 2021 — Answer : The term used to label the energy levels of electrons is, Principle Quantum Numbers (n). Explanation : There are 4 quantum number.
Energy level - definition of energy level by The Free Dictionary energy level n. 1. The energy characteristic of a stationary state of a physical system, especially a quantum mechanical system. 2. The stationary state of a quantum mechanical system. In both senses also called energy state. American Heritage® Dictionary of the English Language, Fifth Edition.
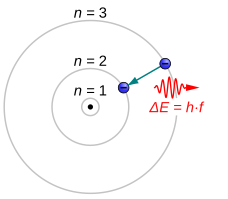
Atomic Energy Levels (video) | Khan Academy These dashed lines represent the different energy levels the electron can have while in the atom. We like representing these energy levels with an energy level diagram. The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time.
Spectroscopic Notation - Stony Brook University The term is the set of levels characterized by a specific S and L. The ground state of Boron has a 2 P 1/2 term. Closed shells always have a 1 S 0 term. Atoms whose outer electrons have l=0,1,2,3,4 are referred to as S, P, D, F, G terms, respectively ( Note that an electron with l=0 is called an s electron; lower case terms refer to individual ...
PDF Electron Energy and Light Key - gardencity.k12.ny.us His model included electrons orbiting the nucleus at specific energy levels. Electrons absorb energy from various sources (electricity) when they move from lower energy levels (ground state) to higher energy levels (excited states). Enerw is released as electrons return to their lower energy levels. 18.
Electrons in Atoms - Practice Worksheet IV - Learning Target 7. What term is used to label the energy levels of electrons? Principal level energy. 8. What letter is used to denote a spherical orbital?
Chapter 5 test Section 5.1 Flashcards - Quizlet The term that is used to label the energy levels of electrons is? S. What letter is used to denote a spherical orbital? Dumbell. All "p" orbital's are _____ shaped? ... Sets with similar terms. Chemistry 101 Exam 1 (topics 1-8) Neyhart. 77 terms. mag998. unit 2: role of the electron. 49 terms. aneshaj01. Chem test 7. 41 terms. lexi_lyles6.
s,p,d,f Orbitals - Chemistry | Socratic An s orbital is spherically symmetric around the nucleus of the atom, like a hollow ball made of rather fluffy material with the nucleus at its centre. As the energy levels increase, the electrons are located further from the nucleus, so the orbitals get bigger. The order of size is 1s < 2s < 3s < …, as shown below.
What term is used to label the energy levels of electrons? The term that is used to label the energy levels of electrons are principle quantum numbers and a valance band refers to the "energy levels in an atom where the electrons that participate in bonding occupy. These energy levels correspond to those of the s and p orbitals of the outermost shell of the atom being considered." Hope this helps! :)
























Post a Comment for "38 what term is used to label the energy levels of electrons"