44 label the reactants and products
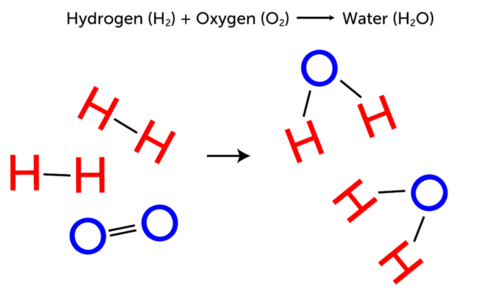
Label the reactants and products, and indicate how many atoms of each ... Label the reactants and products, and indicate how many atoms of each type of element are present on each side of the equation. C_ {2}H_ {6}O (l ) + 3 O_ {2} ( g) →2 CO_ {2} ( g) + 3 H_ {2}O ( g) C 2H 6O(l) +3O2(g) → 2C O2(g)+3H 2O(g) Analysis Reactants are on the left side of the arrow and products are on the right side in a chemical equation. Reactants and Products in Chemical Reactions - dummies Methane and oxygen (oxygen is a diatomic — two-atom — element) are the reactants, while carbon dioxide and water are the products. All the reactants and products are gases (indicated by the g's in parentheses). In this reaction, all reactants and products are invisible. The heat being evolved is the clue that tells you a reaction is taking place.
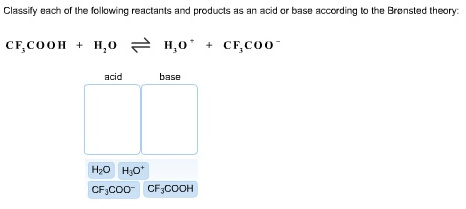
Solved Label the reactants and products (for the forward | Chegg.com Expert Answer 100% (60 ratings) Transcribed image text: Label the reactants and products (for the forward reaction) given the following particulate model. A Conjugate acid BAcid C Base D Conjugate base NH3 (aq) + H2O (L) 근 NH4+ (aq) + OH- (aq) Previous question Next question
Label the reactants and products
[Solved] How does knowing the reactants and products help you classify ... Knowing the reactants and products you can tell to which one of those four classifications a reaction pertains. When two reactants combine into a single product, it is a combination (syntheisis) reaction. When a single reactant yields two or more products, it is a decomposition reaction. Reactants and Products - GeeksforGeeks A chemical equation is a mathematical statement that represents the production of a product from reactants while also indicating the conditions under which the reaction was carried out. The reactants are on the left, and the products are on the right, with one-headed or two-headed arrows connecting them. Consider a reaction. A + B → C + D Here, What are examples of reactants and products in chemistry? Answer (1 of 9): 1. H2 + O2-->2H2O Here h2 and o2 are reactants. and h2o is product 2. FeO2 + SO2-->FeSO4 Here again FeO2 and SO2 are the reactants while FeSO4 is the product
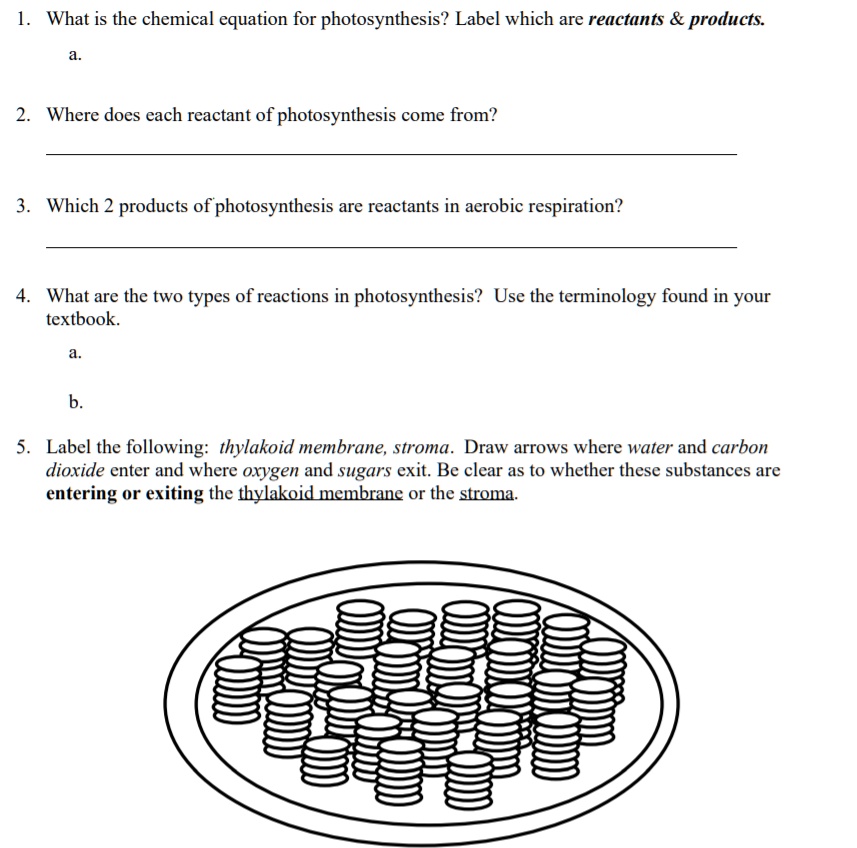
Label the reactants and products. Answered: In the reaction below, label the… | bartleby Q: Label the balanced equation below N2 3 H, € 2 NH, : Reactants : Products : Yetd A: Chemical equation is the way to represent the chemical reaction in terms of chemical formulas of… Q: For the following two reaction equations, write out a complete sentence to show how it would be read… PDF SECTION CHEMICAL REACTIONS 2.4 Study Guide - Ms. Emery's Biology The of the reactants and products determines whether energy will be released or absorbed during a chemical reaction. 7. Before a chemical reaction can start, must be absorbed by the reactants. The amount that must be absorbed to start the reaction is called the. 8. In an exothermic reaction, the products have a bond energy than the reactants. Reactants Products Chemical Reaction Teaching Resources | TpT 5.0. (27) $1.75. Zip. In this worksheet consisting of 30 problems, students will be given the reactants in a chemical reaction and will have to do the following: (1) predict whether or not a reaction will occur, (2) name the type of reaction (synthesis, decomposition, single displacement, or double displacement), (3) write the correct formulas ... PDF Biology Unit 4: Metabolism Photosynthesis & Cellular Respiration 5-2. Be able to name the reactants and products of Photosynthesis. 5-3. Explain how the reactants and products of photosynthesis and respiration relate to each other. Study Guide Photosynthesis 1. Write the overall chemical equation for photosynthesis (label the reactants & the products). H 2 O + CO 2 - - - - - - > C 6 H 12 O 6 + O 2 Reactants ...
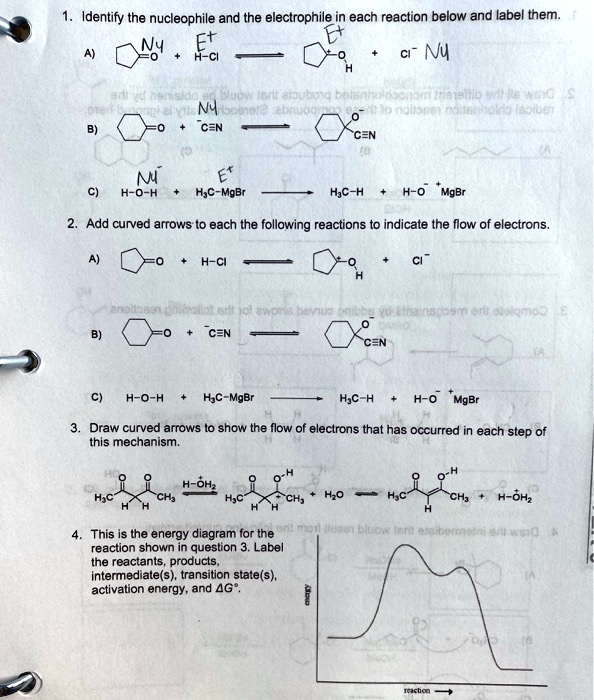
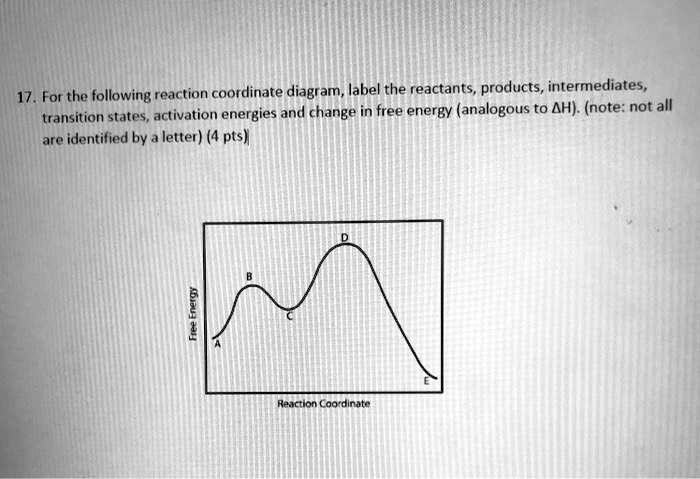
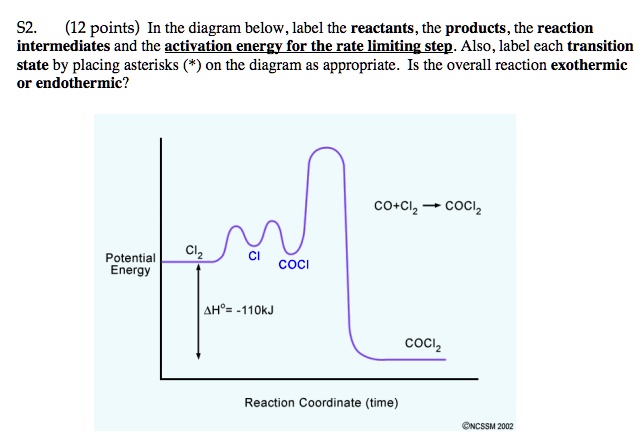
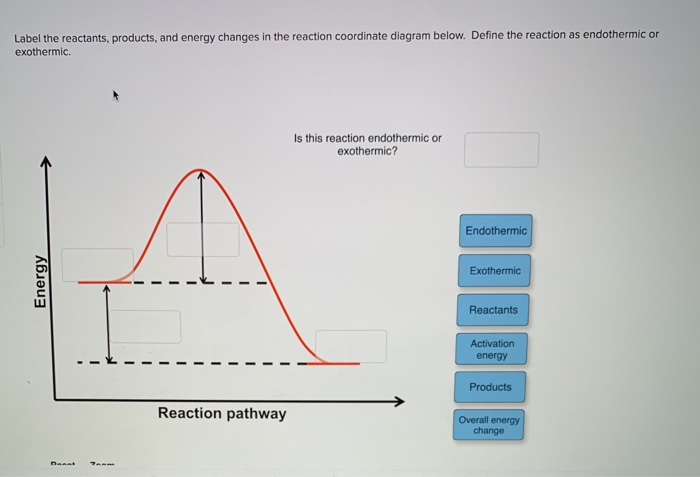
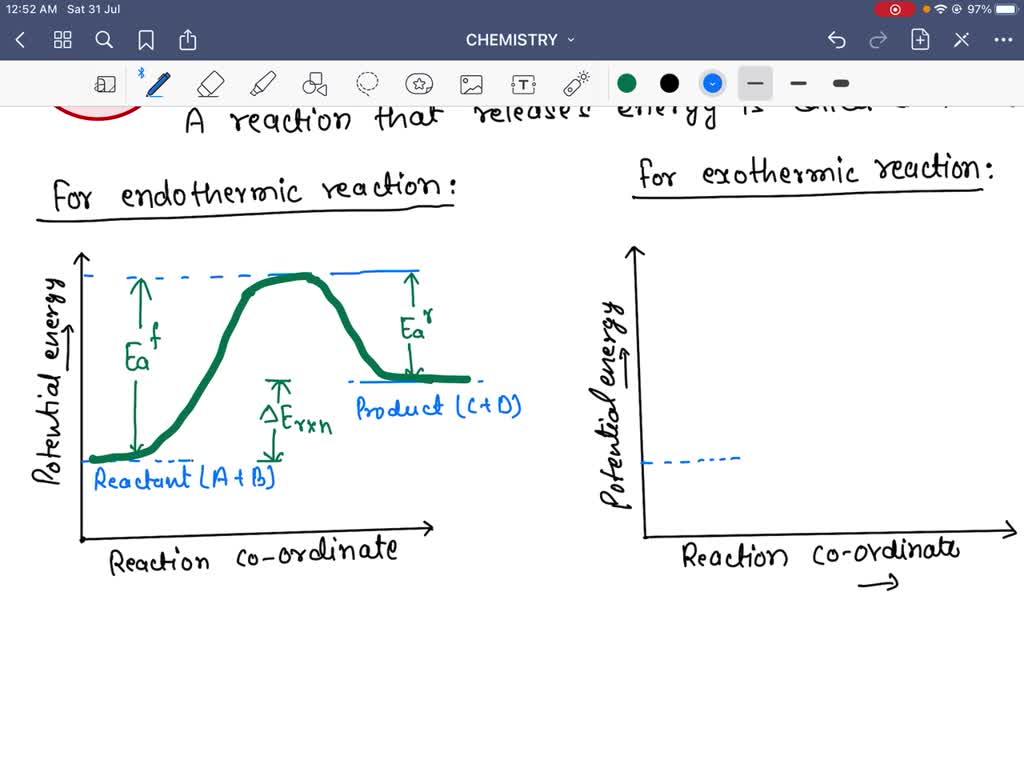
Solved Label the position of the reactants and products and | Chegg.com Expert Answer Transcribed image text: Label the position of the reactants and products and indicate the activation energy and enthalpy of reaction. Energy B D A Reaction Progress Label the position of the reactants and products and indicate the activation energy and enthalpy of reaction. Label the reactants, products, intermediat... - Organic Chemistry - Kunduz Label the reactants, products, intermediates, and transition states. Identify the rate-determining step Drag the appropriate labels to their respective targets. Reset Help 1 2 Group 2 Group 2 1 2 reactants E Group 2 Group 2 Group 2 products intermediate transition state The rate-determining step is Group 1 Reaction progress Label the reactants, products, and intermediates. - Brainly.com Label the reactants, products, and intermediates. - 3312171. LilmayRaegan LilmayRaegan 03/31/2017 Chemistry High School answered Label the reactants, products, and intermediates. 1 See answer Advertisement Exothermic and endothermic reactions - AQA - BBC Bitesize The overall change in energy in a reaction is the difference between the energy of the reactants and products. Exothermic reactions. The diagram shows a reaction profile for an exothermic reaction.
2.4 Flashcards | Quizlet The reactants and products are formed equally; the reaction is in a balanced state. The prefix exo- means "out," and the prefix endo- means "in." What do these prefixes tell you about exothermic and endothermic reactions? Energy goes out of an exothermic reaction and goes into an endothermic reaction. PDF Answer key Label the reactants and products of photosynthesis. Name : Printable Worksheets @ Carbon dioxide Sunlight Oxygen Starch Water Photosynthesis - Reactants and Products g PREVIEW Members, please o his . How can I draw activation energy in a diagram? | Socratic 1. Draw and label a pair of axes. Label the vertical axis "Potential Energy" and the horizontal axis "Reaction Coordinate". 2. Draw and label two short horizontal lines to mark the energies of the reactants and products. 3. Draw the energy level diagram. There must be a hump in the curve to represent the energy level of the activated complex. 4. What Are the Reactants and Products of Photosynthesis? - Reference.com The reactants of photosynthesis are water, light and carbon dioxide, while the products are oxygen and sugars. Cellular respiration occurs in direct synchronicity with this process, using the products of photosynthesis as its reactants and producing its reactants. Photosynthesis occurs in most plant life but may differ in some.
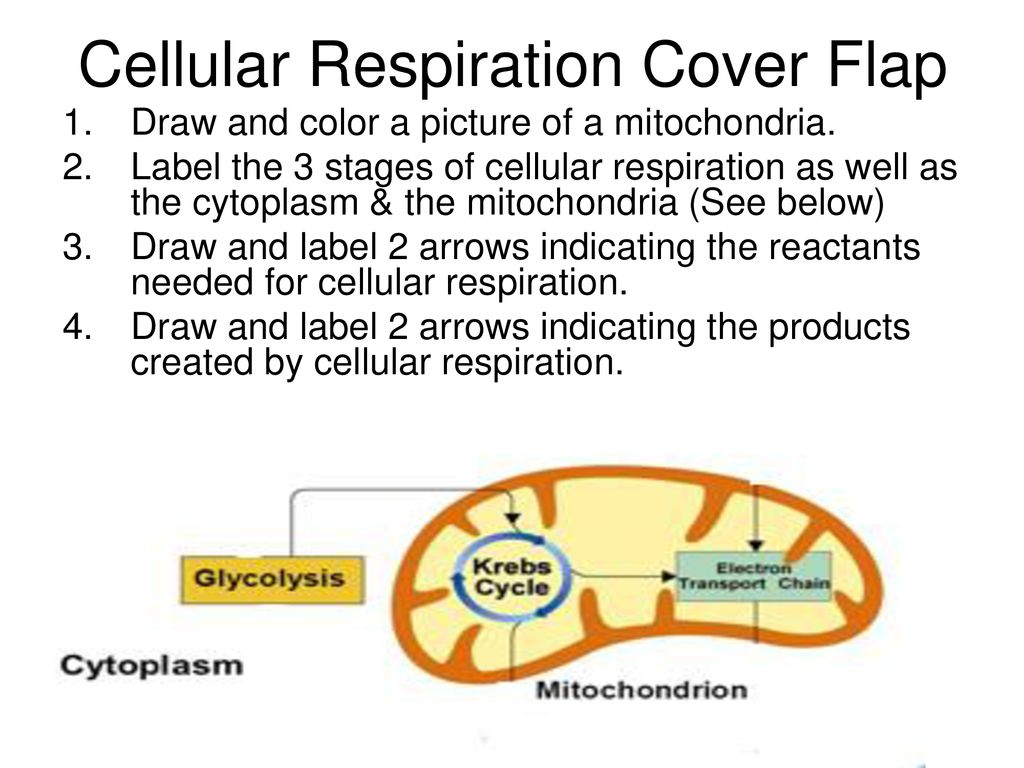
The Reactants And Products Of Cellular Respiration Cellular respiration is the process responsible for converting chemical energy, and the reactants/products involved in cellular respiration are oxygen, glucose (sugar), carbon dioxide, and water. While the exact steps involved in cellular respiration may vary from species to species, all living organisms perform some type of cellular respiration.
7.2 Reactants and products | Chemical reactions | Siyavula Summary. During chemical reactions, materials are changed into new materials with new chemical and physical properties. The materials we start with are called reactants and the new materials that form are called products. During chemical reactions, atoms are rearranged. This requires that chemical bonds in the reactants are broken and that new ...
3.2 Identifying Reactants, Products, Coefficients, and Subscripts in ... Start studying 3.2 Identifying Reactants, Products, Coefficients, and Subscripts in Chemical Equations (AL). Learn vocabulary, terms, and more with flashcards, games, and other study tools.
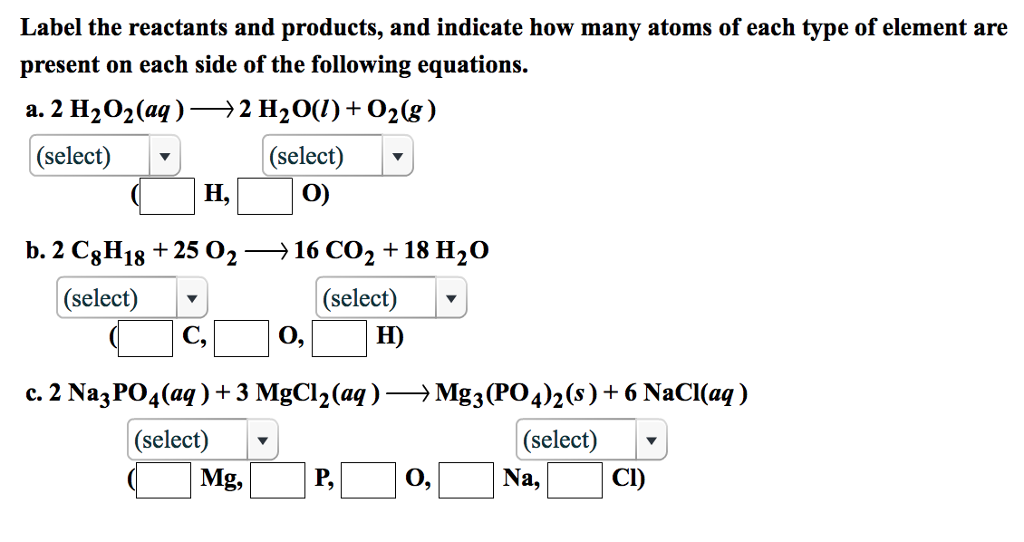
Chemical Equation | Reactants And Products In Chemical Reactions - BYJUS We will first take the maximum number of atoms present on either side of the reaction, that we can find on product side. (Oxygen = 4). Then we multiply the number of oxygen atoms on reactant side by 4, such that the number of oxygen atoms on both sides of the reaction is balanced. The equation becomes F e + 4 H 2 O → F e 3 O 4 + H 2
What are reactants and products? - eNotes.com Reactants and products: these terms are commonly used in relation to chemical reactions, where some chemicals undergo chemical reaction and form new chemical species. The chemical species that ...
SOLVED:Label the reactants and products 0 the enthalpy diagrams for ... Hello here we have to label reactions and the products on the internal P. Tech er diagram for his reaction. Let's start with an atomic reaction. So in an ender thermic reaction the the age yes positive. That's why products have higher entropy and reactors will be lower in. Meanwhile, for the exit ceramic reaction it's the opposite.
Answered: label the reactants and the products… | bartleby ISBN: 9781305957404. Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste. Publisher: Cengage Learning. expand_less. 1 Chemical Foundations 2 Atoms, Molecules, And Ions 3 Stoichiometry 4 Types Of Chemical Reactions And Solution Stoichiometry 5 Gases 6 Thermochemistry 7 Atomic Structure And Periodicity 8 Bonding: General Concepts 9 ...
Reactants and Products | Chemistry for Non-Majors | | Course Hero A reactant is a substance that is present at the start of a chemical reaction. The substance (s) to the right of the arrow are called products . A product is a substance that is present at the end of a chemical reaction. In the equation above, the zinc and sulfur are the reactants that chemically combine to form the zinc sulfide product.
a (3).docx - a. Label the reactants and the products in the... Label the reactants and the products in the following chemical equation. (1 point) CH4+ 2O2 CO2+ 2H2O Co2 and 2H2O are products and Ch4 and O2 are the reactants reactants = CH 4 and 2O 2 products = CO 2 and 2H 2 O
Photosynthesis: Reactants and Products - Visible Body During photosynthesis, light energy converts carbon dioxide and water (the reactants) into glucose and oxygen (the products). 1. Photosynthesis is the process plants use to make their own food. Like all living things, plants need energy to carry out the processes that keep them alive. They get this energy from food.
What are examples of reactants and products in chemistry? Answer (1 of 9): 1. H2 + O2-->2H2O Here h2 and o2 are reactants. and h2o is product 2. FeO2 + SO2-->FeSO4 Here again FeO2 and SO2 are the reactants while FeSO4 is the product
Reactants and Products - GeeksforGeeks A chemical equation is a mathematical statement that represents the production of a product from reactants while also indicating the conditions under which the reaction was carried out. The reactants are on the left, and the products are on the right, with one-headed or two-headed arrows connecting them. Consider a reaction. A + B → C + D Here,
[Solved] How does knowing the reactants and products help you classify ... Knowing the reactants and products you can tell to which one of those four classifications a reaction pertains. When two reactants combine into a single product, it is a combination (syntheisis) reaction. When a single reactant yields two or more products, it is a decomposition reaction.













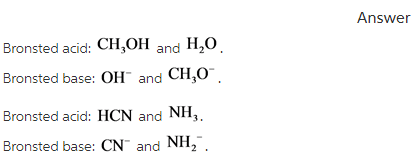










Post a Comment for "44 label the reactants and products"